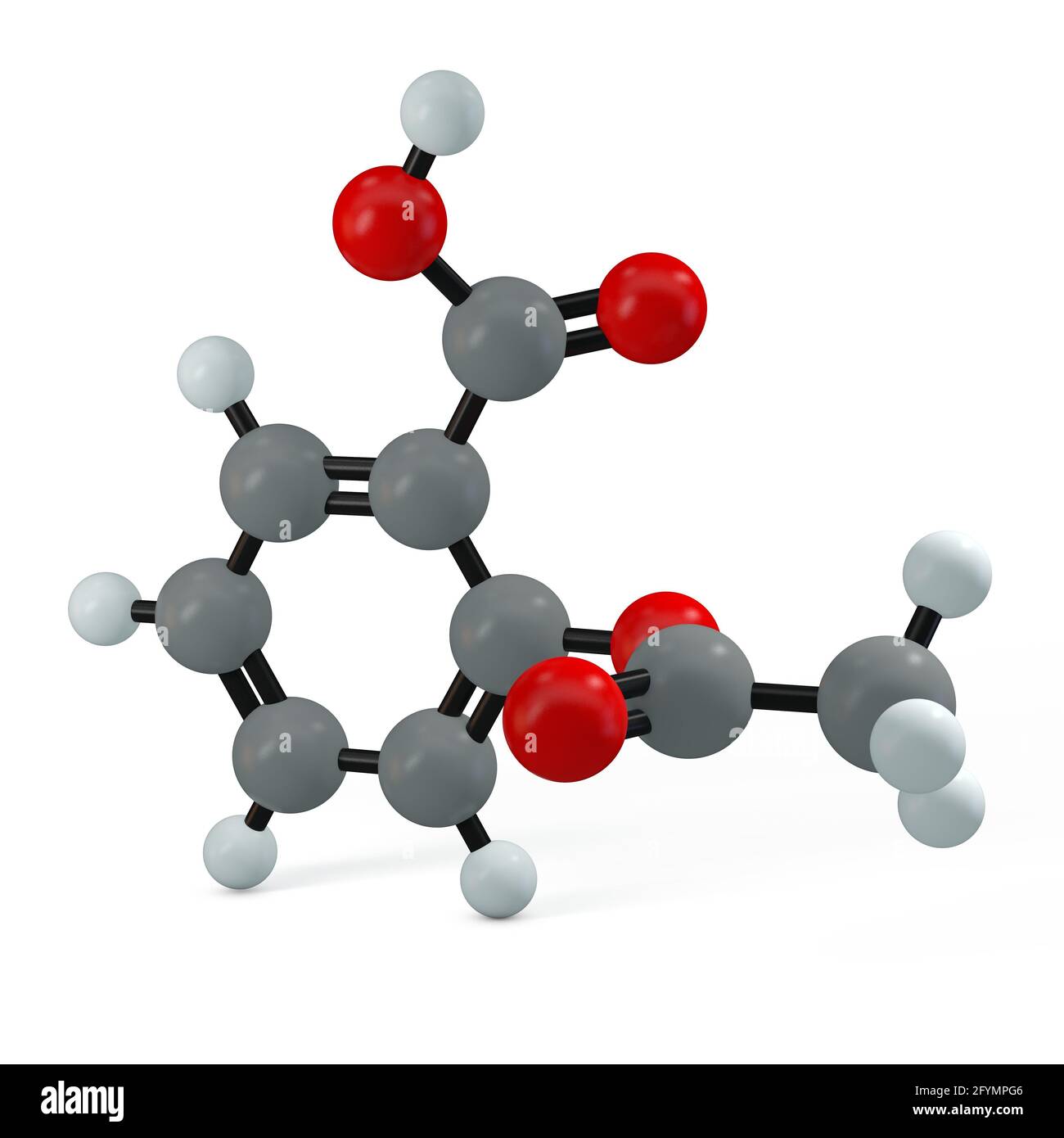
Aspirin Acid Empirical Formula . The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. Calculate the empirical formula for aspirin: The major component of aspirin is acetylsalicylic acid which gives it all these properties. It is also known as aspirin. This is the reason aspirin is also. C 9 h 8 o 4. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen.
from www.alamy.com
Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. Calculate the empirical formula for aspirin: Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. It is also known as aspirin. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. This is the reason aspirin is also. The major component of aspirin is acetylsalicylic acid which gives it all these properties. C 9 h 8 o 4.
Aspirin molecule, illustration Stock Photo Alamy
Aspirin Acid Empirical Formula Calculate the empirical formula for aspirin: Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. C 9 h 8 o 4. Calculate the empirical formula for aspirin: The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. This is the reason aspirin is also. Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. It is also known as aspirin. The major component of aspirin is acetylsalicylic acid which gives it all these properties.
From barmettler38394.blogspot.com
Synthesis Of Aspirin Without Acetic Anhydride Aspirin Acid Empirical Formula Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. Calculate the empirical formula for aspirin: This is the reason aspirin is also. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. C 9 h. Aspirin Acid Empirical Formula.
From revis.bassin.ru
Aspirina formula Despre viața din România Aspirin Acid Empirical Formula C 9 h 8 o 4. Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. The major component of aspirin is acetylsalicylic acid which gives it all these properties. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. Aspirin Acid Empirical Formula.
From proper-cooking.info
Aspirin Molecule Structure Aspirin Acid Empirical Formula Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual. Aspirin Acid Empirical Formula.
From www.alamy.com
Acetylsalicylic acid (aspirin) drug molecule. Skeletal formula Stock Aspirin Acid Empirical Formula C 9 h 8 o 4. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. Aspirin is made of h, o & c,. Aspirin Acid Empirical Formula.
From socratic.org
Question ce7a4 Socratic Aspirin Acid Empirical Formula Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Calculate the empirical formula for aspirin: The major component of aspirin is acetylsalicylic acid which gives it all these properties. The empirical. Aspirin Acid Empirical Formula.
From www.youtube.com
Solubility of Aspirin (Explained) YouTube Aspirin Acid Empirical Formula It is also known as aspirin. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. This is the reason aspirin is also. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The. Aspirin Acid Empirical Formula.
From owlcation.com
A Brief History of Aspirin From Willow Bark to Wonder Drug Owlcation Aspirin Acid Empirical Formula Calculate the empirical formula for aspirin: C 9 h 8 o 4. This is the reason aspirin is also. The major component of aspirin is acetylsalicylic acid which gives it all these properties. Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. It is also known as aspirin. The empirical formula. Aspirin Acid Empirical Formula.
From www.alamy.com
Aspirin molecule, illustration Stock Photo Alamy Aspirin Acid Empirical Formula Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. C 9 h 8 o 4. The major component of aspirin is acetylsalicylic acid which gives it all these properties. This is. Aspirin Acid Empirical Formula.
From www.dreamstime.com
Handwriting Chemical Formula of Aspirin Stock Image Image of studying Aspirin Acid Empirical Formula Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The major component of aspirin is acetylsalicylic acid which gives it all these properties. This is the reason aspirin is also. Calculate the empirical formula for aspirin: It is also known as aspirin. C 9 h 8 o 4. Aspirin. Aspirin Acid Empirical Formula.
From www.alamy.com
Aspirine molecule Stock Vector Images Alamy Aspirin Acid Empirical Formula Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. This is the reason aspirin is also. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. The major component of aspirin is acetylsalicylic acid which gives it all these properties. C. Aspirin Acid Empirical Formula.
From www.meritnation.com
aspirin contains 60 carbon , 4 48 hydrogen , and the rest oxygen Aspirin Acid Empirical Formula Calculate the empirical formula for aspirin: The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. C 9 h 8 o 4. This is the reason aspirin is also. The major component of aspirin is acetylsalicylic acid which gives it all these properties. It. Aspirin Acid Empirical Formula.
From www.coursehero.com
[Solved] Aspirin has a molar mass of 180g/mol. If the epirical formula Aspirin Acid Empirical Formula C 9 h 8 o 4. It is also known as aspirin. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. Calculate the empirical formula for aspirin: The major component of aspirin is acetylsalicylic acid which gives it all these properties. This is. Aspirin Acid Empirical Formula.
From www.alamy.com
Molecule of aspirin Black and White Stock Photos & Images Alamy Aspirin Acid Empirical Formula C 9 h 8 o 4. Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. Calculate the empirical formula for aspirin: The major component of. Aspirin Acid Empirical Formula.
From www.alamy.com
Molecular structure Black and White Stock Photos & Images Alamy Aspirin Acid Empirical Formula Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. Calculate the empirical formula for aspirin: It is also known as aspirin. The major component of aspirin is acetylsalicylic acid which gives it all these properties. Aspirin is made of h, o & c, and was analyzed to contain 60.0%. Aspirin Acid Empirical Formula.
From paperwingrvice.web.fc2.com
What is the chemical equation for the synthesis of aspirin Aspirin Acid Empirical Formula C 9 h 8 o 4. It is also known as aspirin. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. Calculate the empirical formula for aspirin: This is the reason aspirin is also. Aspirin is made of h, o & c, and. Aspirin Acid Empirical Formula.
From mavink.com
Draw The Structure Of Aspirin Aspirin Acid Empirical Formula Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. C 9 h 8 o 4. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. This is the reason aspirin is also. It is also. Aspirin Acid Empirical Formula.
From www.numerade.com
SOLVED A laboratory analysis of aspirin determined the following mass Aspirin Acid Empirical Formula C 9 h 8 o 4. Calculate the empirical formula for aspirin: Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. Acetylsalicylic acid commonly known as aspirin is a prototypical analgesic with the chemical formula c 9 h 8 o 4. It is also known as aspirin. The major component of. Aspirin Acid Empirical Formula.
From www.youtube.com
QuickVideo Calculating the Empirical Formula of Aspirin YouTube Aspirin Acid Empirical Formula Aspirin is made of h, o & c, and was analyzed to contain 60.0% carbon and 35.5% oxygen. The empirical formula represents the lowest whole number ratio of the elements in a molecule while the molecular formula represents the actual formula of the molecule. This is the reason aspirin is also. It is also known as aspirin. The major component. Aspirin Acid Empirical Formula.